Sesame Oil is primarily composed of oleic, linoleic, palmitic, and stearic acids and is used in a variety of cosmetics that come into contact with skin, eyes, hair, and mucous membranes. The oral LD,, of a lipstick containing Sesame Oil (1 0-1 1 %) was greater than 5 g/kg. This ingredient is neither a primary skin nor ocular irritant. A formulation containing Sesame Oil was neither a sensitizer nor a photosensitizer. Although not teratogenic, Sesame Oil increased resorptions and deciduomas in mice. Extracts of sesame seeds were not mutagenic with metabolic activation. On the basis of the information presented in the report, it is concluded that Sesame Oil is safe for use as a cosmetic ingredient.
INTRODUCTION
ESAME OIL IS THE pale yellow oil obtained, largely by pressing methods, from the seeds S of cultivated varieties of the plant, Sesamum indicum. The oil content of the seeds is 45-54% (Estrin et at., 1982a; 1982b; Swern, 1979). In other reviews, the Cosmetic Ingredient Review (CIR) Expert Panel has assessed the safety of four fatty acid components of Sesame Oil: Oleic Acid, Myristic Acid, Palmitic Acid, and Stearic Acid.
The following is a literature review on the chemistry, use, and toxicology of Sesame Oil.
CHEMICAL AND PHYSICAL PROPERTIES
The chemical and physical properties of different samples of Sesame Oil (CAS No. 8008-74-0) vary slightly. However, the description of Sesame Oil used in cosmetics is representative of the oil in general. As specified for use in cosmetics, Sesame Oil must have a characteristic odor with no suggestion of rancidity. The infrared absorption spectrum of the oil must be a close match to the Cosmetic, Toiletry and Fragrance Association (CTFA) spectrum. The Sesame Oil must contain no cottonseed oil. The specific gravity of the oil at 25″C/2S°C must be 0.91 6-0.921. The acid value must not
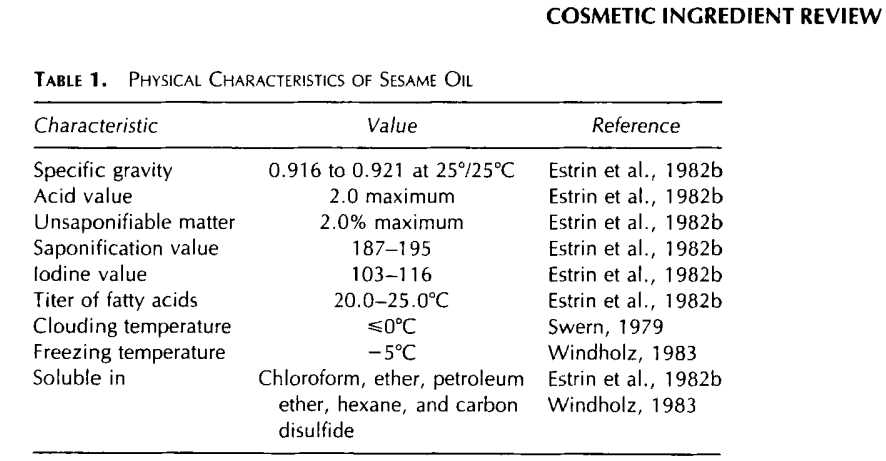
exceed 2.0. The unsaponifiable matter must not exceed 2.0%. The saponification value must be between 187 and 195. The iodine value must be between 103 and 1 16. The titer of fatty acids must be 20.0-25.O"C (Estrin et al., 1982b). Sesame Oil absorbs neither in the ultraviolet A (UVA) nor ultraviolet B (UVB) wavelength range (CTFA, 1992). This information is summarized in Table 1.
Sesame Oil does not cloud at temperatures greater than or equal to 0°C (Swern, 1979). It becomes a solid at about -5°C (Windholz, 1983). Sesame Oil is soluble in chloroform, ether, petroleum ether, hexane, and carbon disulfide. It is slightly soluble in alcohol and is insoluble in water (Estrin et at., 1982b; Windholz, 1983).
The monounsaturated, diunsaturated, and triunsaturated triglycerides of Sesame Oil consist principally of oleic acid and linoleic acid. Other fatty acids are present in smaller amounts (Table 2) (Swern, 1979). In addition to those listed in Table 2, lauric acid, heptadecanoic acid, erucic acid, and lignoceric acid have been detected in Sesame Oil (Sheppard et at., 1978; Swern, 1979). Sesame Oil contains nonsaponifiable
SAFETY ASSESSMENT OF SESAME OIL
substances that are not removed by refining. Sesamol, sesamin, and sesamolin have been detected in Sesame Oil, but have not been detected in other fats. The structures of these compounds are as follows:
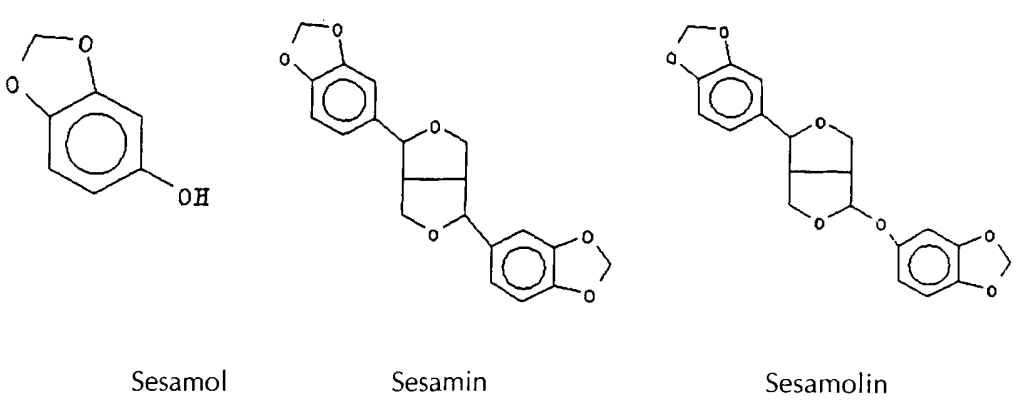
Sesame Oil contains 0.4-1.1 % sesamin, 0.3-0.6% sesamolin, and traces of sesamol. These compounds respond distinctively in colorimetric tests and allow the detection of small amounts of Sesame Oil in other oils even after hydrogenation. The unusual oxidation stability of Sesame Oil before, and even more so after, hydrogenation is attributed to the antioxidant effect of some of these compounds, sesamol in particular.
These compounds are also largely responsible for the synergistic effect of Sesame Oil with pyrethrum insecticides (Budowski and Markley, 1951 ; Swern, 1979).
Sesamol is generated from sesamolin by bleaching, hydrogenation, or other conditions of processing or storage. Sesamol may be removed from Sesame Oil by bleaching or deodorization (Budowski and Markley, 1951 ; Swern, 1979). Researchers recently analyzed a single sample of Sesame Oil and did not detect any sesamol. They concluded that the sesamol previously detected in Sesame Oil might have been a product of the hydrolysis of sesamolin that occurred during the detection procedure (Hayakawa et al., 1987). Sesamol reacts readily in acidic solution with nitrite to produce the C-nitrosated phenol, nitrososesamol. A large amount of sesamol suppressed the N-nitrosation of dimethylamine almost completely by removal of nitrite. A small amount of sesamol in acidic solution removed only a small amount of nitrite, and the nitrososesamol formed catalyzed the N-nitrosation of dimethylamine (Kurechi et al., 1979).
Sesame Oil contains about 0.006-0.19% of plant sterols; these include (3-sitosterol, campesterol, stigmasterol, avenasterol (double bond at C7), and stigmasterol (double bond at C5). Triterpene alcohols have been detected in Sesame Oil at concentrations of about 0.002-0.03%; these include cycloartenol, 24-methylenecycloartenol, a-amyrin, and P-amyrin. Sesame Oil also contains about 0.004% of the 4-methylsterols; these include gramisterol, citrostadienol, and obtusifoliol. Although the concentration range may be misleading because free sesamol interferes with their analysis, tocopherols have been detected in Sesame Oil at concentrations of 0.02-0.05%; these include a-, p-, y-, and &tocopherol. Sesame Oil contains a very small amount of phosphatides, and it contains two pigments, pheophytin A and pheophytin B. Hydrocarbons and aliphatic alcohols have been detected in Sesame Oil at a concentration of about 0.0002%. Five-carbon and nine-carbon straight aldehydes and acetylpyrazine are responsible for the odor and taste of Sesame Oil (Swern, 1979).
Zoncentrations of about 0.8-2.5 pg/g of the 1,2-dicarbonyl compounds, glyoxal, methyl glyoxal, and diacetyl have been detected in Sesame Oil autooxidized by heating. These compounds are mutagens for Salmonella typhimuriurn TA100 without metabolic activation (Hirayama et al., 1984).
Aflatoxin B, was not detected in 10 samples of refined Sesame Oil. It was detected in 10 samples of raw Sesame Oil at concentrations of 0.02-0.15 ppm (Sengupta and Roy, 1983).
USE - Cosmetic
Sesame Oil is used in cosmetics as a solvent, skin conditioning agent, and hair conditioning agent (Nikitakis, 1988). Cosmetic products containing Sesame Oil may be applied to or may come in contact with skin, eyes, hair, nails, and mucous membranes.
Product formulations containing Sesame Oil may be applied as many as several times a day and may remain in contact with the skin for variable periods following application.
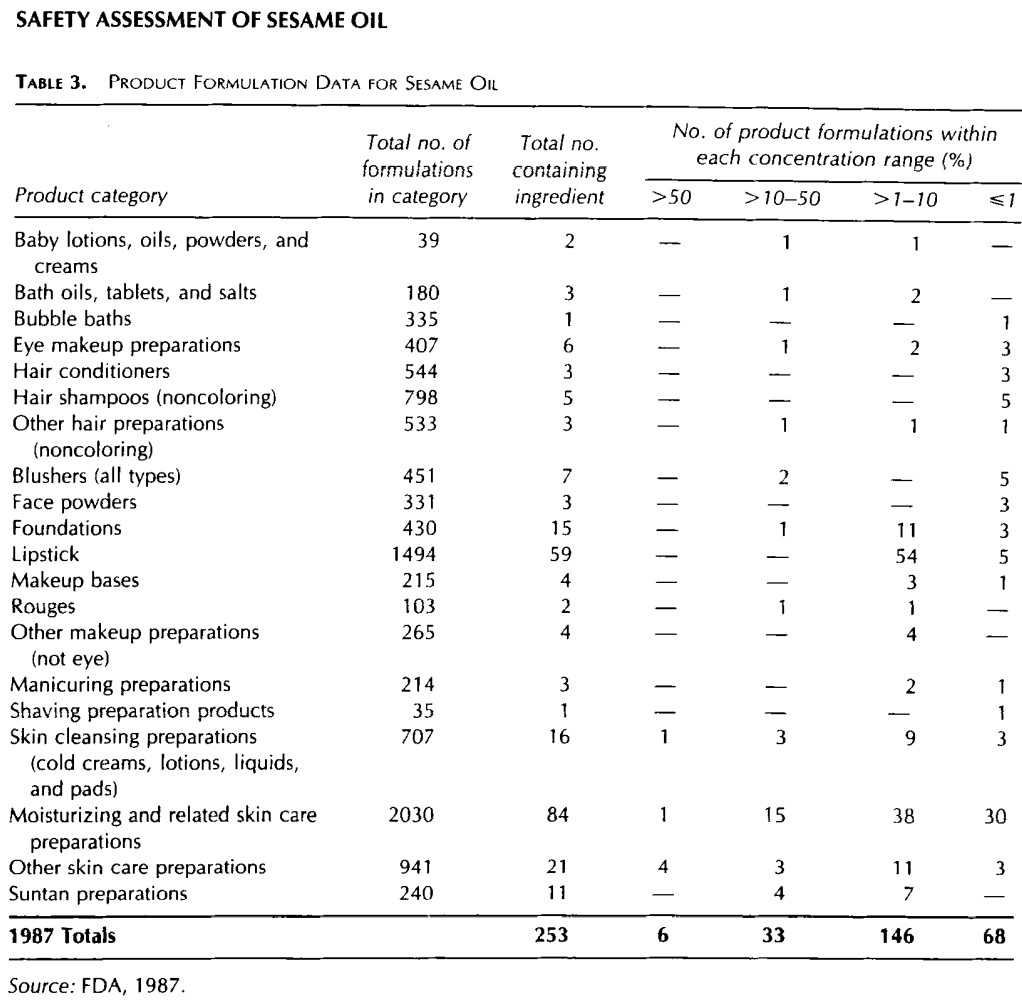
Daily or occasional use may extend over many years (FDA, 1987). Product types and the number of product formulations containing Sesame Oil reported voluntarily to the Food and Drug Administration (FDA) in 1987 are presented in Table 3. Sesame Oil was reported to be used in 375 formulations in 1991 (FDA, 1991 ). Voluntary filing of this information by cosmetic manufacturers, packagers, and distributors conforms to the prescribed format of preset concentration ranges and product types as described in the Code of Federal Regulations (21 CFR 720.4). Some cosmetic products are supplied by the manufacturer at less than 100% concentration and, therefore, the value reported by the cosmetic formulator may not necessarily reflect the true concentration of the finished product; the actual concentration in such a case would be a fraction of that reported to the FDA.
The fact that data are only submitted within the framework of preset concentration ranges provides the opportunity for overestimation of the actual concentration of an ingredient in a particular product. An entry at the lowest end of a concentration range is considered the same as one entered at the highest end of that range, thus introducing the possibility of a 2- to 10-fold error in the assumed ingredient concentration. In 1987, Sesame Oil was reported as an ingredient in 253 cosmetic formulations at concentrations ranging from C1% to >50% (Table 3) (FDA, 1987).
Noncosmetic
Sesame (seed or oil not specified) is a generally recognized as safe (GRAS) food additive permitted for direct addition to food for human consumption (21 CFR 182.1).
Sesame Oil may be used as an indirect food additive; it can be used in the production of resinous and polymeric coatings used as the food-contact surfaces of articles intended for producing, manufacturing, packing, processing, preparing, treating, packaging, transporting, or holding food (21 CFR 175.300).
Sesame seeds and Sesame Oil have been consumed by humans for thousands of years. Crude expressed Sesame Oil can be used as a food with little or no refining. It is consumed primarily as a cooking and salad oil and is hydrogenated for margarine and shortening use. Sesame Oil is an important flavor in Asian food (Budowski and Markley, 1951; Swern, 1979). Sesame seed products can be fed to poultry and livestock (Budowski and Markley, 1951 ; Rossoff, 1974).
Sesame Oil has been evaluated in the FDA over-the-counter (OTC) drug review program, but has not been categorized by any Advisory Review Panel (Table 4)
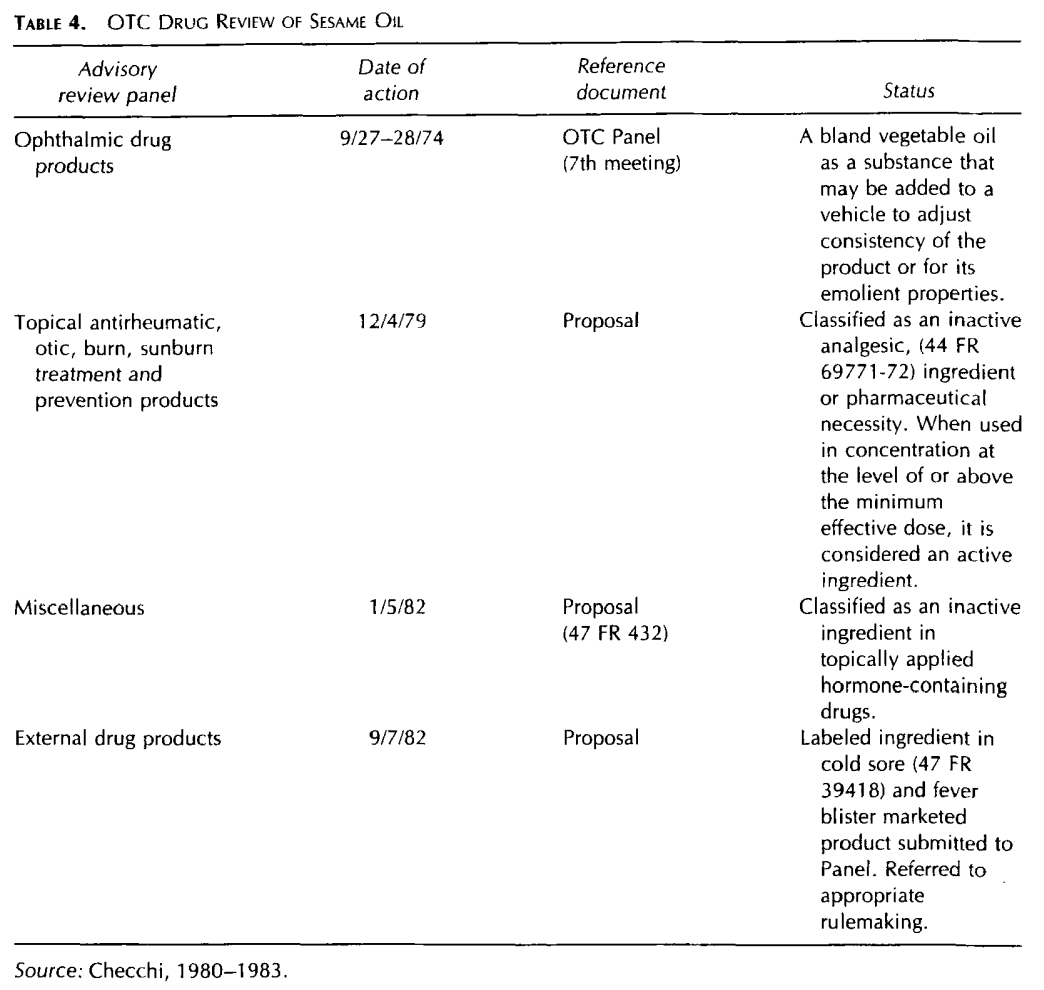
(Checchi, 1980-1 983). Sesame Oil is on the FDA 1982 list of inactive ingredients for approved prescription drug products (Brown, 1983).
Sesame Oil has been used in pharmaceuticals as a carrier for fat-soluble substances and as an emollient (Swern, 1979). It has been used as a vehicle for intramuscular injections in humans (Gosselin et al., 1976) and in animals (Rossoff, 1974). Sesame Oil is a laxative when consumed in large amounts (Gosselin et al., 1976).
Sesame Oil has been used in the manufacture of soap. It has also been used as a synergist for pyrethrum pesticides (Swern, 1979). Sesame Oil has been used as a pyrethin synergist particularly against lice on cattle at a concentration of 0.7-1 .O% and against lice on premises at a concentration of 12% (Rossoff, 1974).
ABSORPTION AND DISTRIBUTION
Polymerized Sesame Oil was applied to the depilated abdomens of eight albino rats each day for 30 days (D’Souza and Ramaiah, 1980). Nonpolymerized Sesame Oil was applied to the abdomens of 8 control rats each day for 30 days. At the end of the study, the rats were killed, and the skin and subcutaneous fat were removed for histopathologic evaluation. No differences were found between control (treated with nonpolymerized Sesame Oil) and treated (treated with polymerized Sesame Oil) rats in the gross appearance of the skin. The average thickness of the epidermis of the control rats was significantly greater than that of the treated rats. No other significant histologic differences were observed.
Polymerized Sesame Oil was applied to one side of the depilated abdomens of 8 albino rats each day for 30 days (D’Souza and Ramaiah, 1980). One side of the depilated abdomens was not treated. After 30 days, the animals were anesthetized, and the skin sites were removed from the abdomens and homogenized for protein, enzyme, and lipid analysis. The most important biochemical changeobserved in the treated skin was the increase in hormonal steroids. The activity of glucuronidase was increased, the activity of glucosaminidase was decreased, the amount of 3-oxosteroids (with a double bond at C4) was increased and free and conjugated 17-ketosteroids, mucopolysaccharides, and glycoproteins of the skin were decreased.
The incorporation of [l4C1acetate into lipids and the conversion of [3H]progesterone to hydrocortisone in treated and control rat skin was investigated in vitro (D’Souza and Ramaiah, 1980). The incorporation of radioactivity into sterols was increased and into diacyl glycerols and esters was decreased in treated rat skin. The conversion of [3H]progesterone to hydrocortisone was significantly greater in treated rat skin than in
control rat skin.
A single 0.5 ml/kg dose of sterile Sesame Oil mixed with a small amount of ['4C]gIyceryl trioleate (final specific activity was 37 pCi/ml) was given intramuscularly in the thigh to 16 Beagle dogs, and 2 dogs were killed for necropsy 1,2,4, 6, 14,21, 28, and 35 days after the injection (Svendsen and Aaes-jorgensen, 1979). About 70% of the injected radioactivity was detected in the thigh 1 and 2 days after the injection. After 35 days, about 50% of the radioactivity was still present at the injection site. Radioactivity in the heart, liver, and lungs increased over the 35 day observation period. The greatest amountsof radioactivity were found in the heart, liver, and lungs and were0.042,O. 15, and 0.055% of the dose, respectively. The highest concentration of radioactivity was in the iliac lymph nodes and was detected 28 days after the injection. These lymph nodes contained the greatest amount of radioactivity of any of the lymph nodes sampled.
Sesame Oil was absorbed by the regional lymph nodes, but not to the extent that pulmonary oil microembolism occurred. Two groups of 3 male and 3 female Beagle dogs were given intramuscular injections of 0.45 and 1 .O ml/kg of Sesame Oil in alternating thighs once a week for 6 months (Svendsen and Aaes-Jorgensen, 1979). The last dose given these animals was sterile Sesame Oil mixed with a small amount of ['4Clglyceryl trioleate (final specific activity was 37 kCi/ml). A third group was the untreated controls.
Two male and 2 female dogs were given 0.45 ml/kg Sesame Oil once a week for 6 months and were used to study the reversibility of oil-induced changes. Hematologic, clinical chemical, and urinary parameters were evaluated.
Two dogs from each group were killed for necropsy at each time period of 1,2, and 6 days after the injection of 14C-labeled oil mixture. Two dogs from the group used to study the reversibility of oil-induced changes were killed for necropsy 1 and 3 months following the last injection.
Large oil deposits, a slight yellow color, and fibrosis were observed at the injection site. The iliac lymph nodes were greatly enlarged in dogs of both treatment groups, but to a greater extent in the dogs of the high-dosagegroup. The iliac lymph nodes contained numerous oil cysts. They were still enlarged 1 and 3 months after the last injection. Oil microembolisms following lymph vessel absorption of the oil were observed in the lungs of all treated dogs necropsied 1, 2, and 6 days after the last injection. These were most severe in the dogs of the high-dosage group. In addition, focal interstitial oil accumulations were associated with interstitial infiltration of polymorphonuclear leukocytes and macrophages.
Focal hemosiderosis was noted in the lymph nodes of dogs in the high-dosage group. Oil microembolisms in the lungs, oil cysts in the iliac lymph node, and hemosiderosis were observed 1 and 3 months after the last injection. However, at 3 months, the size and number of oil cysts had decreased and the lymphoid tissue had partially regenerated. Small oil cysts were still observed at the injection site after 3 months. The amount of radioactivity in visceral organs and lymph nodes of the low-dosage group was similar to that observed after an acute dose of Sesame Oil.
Greater amounts were observed after the high dosage. The iliac lymph nodes contained up to 4.4% of the initial dose of radioactivity. The amount of radioactivity at the injection site was “relatively low” 1 day after the injection of the radioactive oil mixture, and did not decrease over the next 5 days.
Groups of 2 male Wistar/Af/Han 67/Mol,SPF mice each were given 0.5 ml sterile Sesame Oil mixed with a small amount of ['4C]glyceryl trioleate (final specific activity was 37 kCi/ml) intramuscularly in the thigh, and the mice were killed 7 and 16 hr and 1, 3, 5, 7, 10, 14, 21, 28, 35, and 49 days after the injection (Svendsen and Aaes-Jorgensen, 1979). The amount of radioactivity in the thigh declined exponentially with time and the half-life of the radioactivity was about 9 weeks.
Three groups of 5 male Wistar rats each were given 0.25 and 0.5 ml of Sesame Oil intramuscularly in alternating thighs 3 times a week for 5 weeks (Svendsen and Aaes-Jorgensen, 1979). The rats were killed for necropsy 3 days after the last injection. Large amounts of oil, mostly present as oil cysts, were present at the injection site and the amount of oil observed was dose-dependent. A few small oil cysts were observed in the iliac lymph nodes of the low-dosage animals. Greater numbers and larger cysts were observed in the lymph nodes of the high-dosage animals. Pulmonary oi I microembolism after lymph vessel absorption of the oil was observed only in the high-dosage rats.
Groups of 2 rabbits each received 0.5 ml Sesame Oil intravenously and were killed for necropsy 4 h, 2 and 4 days, and 1, 2, 3, and 4 weeks after the injection (Svendsen and Aaes-Jorgensen, 1979). Two untreated rabbits served as controls. Seven rabbits died during the study and were replaced. Numerous focal pulmonary hemorrhages were noted. Pulmonary oil microembolisms wereobserved 4 h, 2 and 4 days, and 1 and 2 weeks after the injection. At 2 weeks, Sesame Oil was present in the pulmonary interstitiurn and was accompanied by inflammatory cellular infiltration.
Five rabbits received 1 .O mikg Sesame Oil stained with Sudan black intramuscularly in alternating thighs 3 times a week for 2 weeks (Svendsen and Aaes-Jorgensen, 1979). The rabbits were killed for necropsy 3 days after the last injection. The thighs of the rabbits were markedly black and contained cysts filled with oil. Black spots also were present in the iliac lymph nodes. Focal hyperemia and a few focal hemorrhages were observed in the lungs. Small oil cysts in the iliac lymph node and pulmonary microembolism were observed in one rabbit.
GENERAL BIOLOGY
Blood Effects
Kurechi et al. (1 980) found that sesamol transformed hemoglobin A into methemoglobin. Purified oxyhemoglobin and a suspension of erythrocytes were prepared.
Sesamol was added to the suspension of erythrocytes, 1:4, and concentrations of oxyhemoglobin and methernoglobin were determined by the extinction coefficient at 576 and 630 nm. Ninety percent of the oxyhemoglobin was oxidized to methemoglobin after 25 min.
Enzymology
Male Swiss albino mice were intraperitoneally injected with 0.1 ml of 160 mg/kg sesamol and other synergists, 4 mice per group. Animals were killed 1 h later, and livers were removed for preparation of microsomal suspension. Dimethylaminopyrine and hexobarbital substrate assays were used to determine the drug-hydroxylating activity of the prepared fractions. Sesamol weakly inhibited the hydroxylation of the tested substrates: dimethylaminopyrine, 88% of control; hexobarbital, 83% of control (jaffe et al., 1968).
Fujii et al. (1970) found sesamol and sesamin to have a moderate effect on the prolongation of hexobarbital narcosis and zoxazolamine paralysis in mice as compared to controls. Unlike many methylenedioxyphenyl compounds, however, sesamol did not form complexes with cytochrome P-450 in the liver of mice intraperitoneally injected with 150 mg/kg sesamol daily for 3 days (Fennel1 and Bridges, 1979). Dahl and Brezinski (1 985) studied the effects of methylenedioxyphenyl compounds on nasal as well as hepatic microsomal cytochrome P-450-dependent hexamethylphos phoramide (HMPA) N-demethylase.
Microsomes were prepared from nasal and hepatic tissues removed from healthy male New Zealand white rabbits. Spectra were then recorded using 10 p,I of a 700 mM solution of each of the test compounds added to the microsomal fraction and then compared to a reference spectrum of 0.77 pM nasal microsomal cytochrome P-450. Nasal microsomes in the presence of sesamol formed a type Ill spectrum upon the addition of NADH. However, after dithionite was added, the spectrum became that typical for the carbon monoxideP-450 complex.
ANIMAL TOXICOLOGY
Oral Acute (CTFA, 1989).
Subchronic
Ten male Charles River CD-1 mice received 0.1 ml Sesame Oil orally 5 times per week for 13 weeks (Anton et al., 1974). Ten saline-treated mice served as controls. The mice were killed 1 h after the final dose. The concentration of norepinephrine was significantly reduced in the brain, heart, and spleen. Concentrations of serotonin and 5-hydroxyindoleacetic acid in the brains of treated mice were not significantly different from those in the brains of control mice.
The LD,,of a lipstick containing 10-1 1 % Sesame Oil was greater than 5 g/kg in rats
Subcutaneous
Groups of 9-10 male and female C3HfHej mice were given 0.05 ml Sesame Oil subcutaneously on the fourth postnatal day and were killed at about 210 days of age (Blizard, 1978). Control groups of mice were untreated and were not handled. The weights of the brain and body weights of the treated mice were significantly reduced.
Randomly bred mice were derived from a systematic cross of eight inbred mouse strains (Blizard, 1978). Groups of 13-30 male and female mice were injected subcutaneously with 0.05 ml saline or 0.05 ml Sesame Oil on the fourth postnatal day. The animals were killed at 42 days of age. No neonatal treatment effects were observed. All groups of male mice had significantly greater body weights than any groups of female mice.
Intravenous
Groups of 3-5 New Zealand White rabbits were given 0.50 to 1.25 ml/kg Sesame Oil intravenously in a marginal ear vein (Svendsen and Aaes-Jorgensen, 1979). The animals were observed for 24 h after the injection and the LD, for rabbits was 0.74 mlikg.
Dermal Irritation
The dermal LD, of Sesame Oil was greater than 2 g/kg in rabbits (CTFA, 1989). The primary skin irritation of 100% Sesame Oil was investigated in a single-insult occlusive patch test procedure. Four studies were performed with nine rabbits each. The skin of the animals was observed 2 and 24 h after patch removal. The primary irritation indices (PII) (of a maximum possible PI1 of 8.0) in the 4 studies were 0.22 (CTFA, 19781, 0.00 (CTFA, 1975a), 0.1 1 (CTFA, 1975b), and 0.0 (CTFA, 1975~).
Eight serial open patches of 100% Sesame Oil were applied to the nonabraded skin of 9 rabbits (CTFA, 1972a). The skin was observed at 24,48, and 72 h after application.
The PI1 (of a maximum possible PI1 of 8.0) was 0.83.
Ocular
Undiluted Sesame Oil was instilled into the conjunctival sac of three rabbits; the eyes were not rinsed (CTFA, 1972b). Theeyes wereobserved 1 day after instillation. No ocular irritation was observed; the Draize eye irritation score (of a maximum possible eye irritation score of 1 10) was 0.
Undiluted Sesame Oil was instilled into the eyes of six rabbits; the eyes were not rinsed (CTFA, 1975d). The eyes were observed 1, 2, and 3 days after instillation.
Minimal eye irritation was observed; the Draize eye irritation score (of a maximum possible eye irritation score of 11 0) was 3 on day 1, 2 on day 2, and 1 on day 3.
A lipstick containing 10-1 1 % Sesame Oil was negative for ocular irritation in 6 test animals (CTFA, 1989).
Teratology
Doses of 4 ml Sesame Oil were administered by gavage to 5 pregnant Wistarderived rats daily on days 6-1 0 of gestation, and pregnancies were terminated on day 20 (Beaudoin, 1981 1. The Sesame Oil might have been embryolethal, but was not teratogenic. The 16.2% rate of resorptions with Sesame Oil was greater than the 5.3% rate observed in untreated controls in the laboratory colony over the previous 20 yrs. No surviving fetuses were malformed.
A 0.4 ml dose of Sesame Oil was administered intraperitoneally to 41 virgin female (SEC x C57BL)Fl mice and, 5 days later, the female mice were mated with untreated male (C3H X C57BL)Fl mice (Generoso et al., 1984). The 36 pregnant female mice were killed for uterine analysis 12-13 days after mating. The Sesame Oil induced a marked increase in the incidence of deciduomas.
Twenty-eight male and 32 female BALB/c mice received weekly subcutaneous injections of 0.05 ml Sesame Oil beginning at 3-5 days of age (duration of study unspecified; presumably, for lifetime of animals) (Szepsenwol et al., 1979a). These mice developed and reproduced normally. The offspring of these mice developed
normally and did not require foster nursing.
MUTAGENlClTY
Chloroform-methanol and water extracts of sesame seeds were tested for mutagenicity in the Ames Salmonella/mammalian microsome mutagenicity test (Rockwell and Raw, 1979). The sesame seed extracts were not mutagenic for Salmonella strains TA98 and TA100 upon metabolic activation.
CARCINOGENICITY
Sesame Oil has been used frequently in carcinogenicity studies as a vehicle for the chemical agent under test. In many cases, the published literature does not provide
results for untreated or historical controls. The studies described in this section do include untreated or historical controls. Many other studies, with or without controls, are available in the literature.
In abstracts, Szepsenwol et al. (1 979a,b,c) reported a study in which 28 male and 32 female BALB/c mice received weekly subcutaneous injections of 0.05 ml Sesame Oil beginning at 3-5 days of age (duration of study unspecified; presumably, for lifetime of animals). There were 150 male and 180 female untreated controls. All mice developed normally. Fewer than 1 % of the untreated female micedeveloped mammary cancer. Of the 32 Sesame Oil-treated female mice, 16 (50%) developed mammary adenocarcinomas at ages older than 21 3 days. Sarcomas did not develop at the site of injection of the Sesame Oil. Three to four neoplasms developed at the same time in most of the mice. Multiple metastases of the neoplasms were observed in the mediastinum and lungs.
Mice with mammary carcinoma had large ovaries with follicles in various stages of development, atretic follicles, and large corpora lutea. The researchers theorized that the injected Sesame Oil stimulated the production of luteinizing hormone and prolactin, which are primary factors in mammary carcinogenesis. In a similar study, in which BALB/c mice either recpived weekly subcutaneous injections of Sesame Oil or were untreated, no adrenal gland neoplasms were observed (Szepsenwol et al., 1982).
Fifteen female Sprague-Dawley rats were injected subcutaneously with 0.2 ml Sesame Oil daily for 87 days beginning at 50 days of age (Huggins and Grand, 1963).
The animals were killed for necropsy after 280 days. Oil accumulated in pockets at the injection site and persisted for more than 6 months after the injections were discontinued. No sarcomas wereobserved in any Sesame Oil-treated animal. No sarcomas have ever been observed in untreated rats in this laboratory.
Doses of 0.5 ml Sesame Oil were given intraperitoneally to pregnant HailCR mice on days 11, 13, and 15 of gestation (Bulay, 1970; Bulay and Wattenberg, 1971). In one experiment, the mice were allowed to deliver spontaneously, and the offspring were killed for necropsy at 1 yr of age. There were 10 female and 7 male offspring from the Sesame Oil-treated group, and there were 7 female and 6 male offspring from an untreated control group. In the Sesame Oil-treated group and the untreated group, 11.7% and 23.0% of the animals had pulmonary adenomas, respectively. In a second experiment, the mice were delivered by cesarean section and were nursed by foster mothers. The 1 7 female and 6 male offspring from the Sesame Oil-treated group and the 7 female and 8 male offspring from the untreated control group were killed for necropsy at 8 monthsof age. In the Sesame Oil-treated group and the untreated group, 13.0% and 6.6% of the animals had pulmonary adenomas, respectively.
Fifteen female BALB/c mice were injected subcutaneously with 0.02 ml Sesame Oil daily for 5 days beginning within 36 h after birth (Jones and Bern, 1979). There were 35 untreated controls. The mice were killed at a mean age of 22.0-22.3 months for necropsy. No vaginal or cervical lesions were observed in the Sesame Oil-treated animals. Genital tract epithelial abnormalities, which were not hyperplastic or neoplastic lesions, were observed in two untreated mice. Mammary neoplasms were not observed in any mice. All pituitary glands were morphologically normal in appearance.
Twelve male and 13 female strain R9 guinea pigs were given 2 injections of 1 ml Sesame Oil subcutaneously with 30 days between injections (Blumenthal and Rogers, 1962). Three of these animals were observed for as long as 679 days after the second injection. There were 4000 strain R9 and 2000 strain R7 historical controls. No subcutaneous or intramuscular neoplasms were observed in litters of the treated guinea pigs or in any of the controls.
Naturally occurring antioxidants, including sesamol, and synthetic antioxidants were compared for their production of gastric lesions in male F344 rats. Feed treated with 1 and 2% sesamol was administered to a group of 6-week-old rats for 4 weeks. Body weights were reduced 10-15% compared to controls. Rats receiving the 2% sesamol, but not 1 % feed, had large, usually single, ulcers surrounded by a thickened epithelium in the nonglandular area of the stomach. The greater concentration of sesamol also induced moderate hyperplasia in the prefundic region and severe hyperplasia in the central region of the nonglandular gastric epithelium, whereas the lower concentration failed to induce any hyperplasia of this region. Because of the difference in results between the two concentrations, the authors suggested that the action of 2% sesamol may be purely toxic (Hirose et al., 1987). In a similar study, Hirose et al. (1 990) found that 2% sesamol induced squamous cell carcinoma of the forestomach in both sexes of B6C3F1 mice and F344 rats and papillomas in both sexes
of the rat.
CLINICAL ASSESSMENT OF SAFETY
Dermal Irritation and Sensitization and Photosensitization
Predictive
Sesame Oil, 0. I mg, was applied to the abraded skin of 5 white male and female subjects each day for 3 days within an aluminum chamber over the scratches (Drill and Lazar, 1977). Water-permeable, nonocclusive tape was used to seal the chambers to the skin. Irritation reactions were scored on a 0-4 scale 72 h after the beginning of the study. Sesame Oil had a low irritancy potential; the irritation score was 0-0.4.
An occlusive patch containing full-strength Sesame Oil plus Butylated Hydroxyanisole (BHA) was applied to the skin of 20 subjects for 24 h (CTFA, 1972~). No erythematous reactions were observed in any of the subjects in this single-insult patch test procedure.
A repeated-insult patch test with 0.4 ml/patch of a lotion containing 8% Sesame Oil was conducted using 201 subjects (Hill Top Research, 1976a). Occlusive induction patches were applied to the backs of the subjects three times a week for 3 weeks. The first induction patch was applied for 48 h, and the skin reaction was scored at patch removal and at 96 and 168 h after application. Patches 2-9 were applied for 24 h to a site adjacent to the first patch, and skin reactions were scored at patch removal. A 48 h challenge patch was applied to a previously untreated site after a 2 week nontreatment period, and the skin reactions were scored at patch removal and at 96 and 168 h after application. There were no reactions to the product.
SAFETY ASSESSMENT OF SESAME OIL 273
A repeated-insult patch test with 0.4 ml/patch of a cream containing8% Sesame Oil was conducted using 11 2 subjects (Hill Top Research, 1977). Occlusive induction patches were applied to the upper arms of the subjects three times a week for 3 weeks. Induction patches were applied for 24 h, and each skin reaction was scored just prior to the next patch application. Patch 9 was scored 48 h after application. A 24 h challenge patch was applied to a previously untreated site after a 2 week nontreatment period, and skin reactions were scored at 48 and 96 h after application. Fifteen subjects had at least one reaction at induction or challenge. Of 957 induction readings, there were 22 1 + reactions and five 2+ reactions. Of 220 challenge readings, there were three 1 + reactions and one 2+ reaction. The product was essentially nonirritating.
A repeated-insult patch test with approximately 0.1 mI/patch of a facial mask containing 8% Sesame Oil was conducted using 99 subjects (CTFA, 1976). Occlusive induction patches were applied to the backs of the subjects three times a week for 3 weeks. Induction patches were applied for 24 h, and each skin reaction was scored (on a scale of 0-4) just prior to the next patch application. Patch 9 was scored 24 h after application. A 24 h challenge patch was applied to a previously untreated site after a 3% week nontreatment period, and skin reactions were scored at 48 and 72 h after application. Sixty-two subjects had at least 1 reaction at induction or challenge. Of 891 induction readings, there were 184 -C (barely perceptible) reactions and 84 1 + (mild) reactions. Of 198 challenge readings, there were 1 1 2 reactions and 7 1 + reactions.
A repeated-insult patch test with 0.4 mI/patch of a facial moisturizer containing 14.3% Sesame Oil was conducted using 109 subjects (Hill Top Research, 1976b).
Occlusive induction patches were applied to the backs of the subjects 3 times a week for 3 weeks. The first induction patch was applied for 24 h, and the skin reaction was scored 20 min and 48 and 120 h after patch removal. Patches 2-9 were applied for 24 h to a site adjacent to the first patch, and skin reactions were scored just prior to the next patch application. The reaction to patch 9 was scored 48 h after removal. A 24 h challenge patch was applied to a previously untreated site after a 2 week nontreatment period, and skin reactions were scored 20 min and 48 and 120 h after patch removal.
One subject had a 2+ reaction to the ninth induction patch. Two other subjects had reactions at the 20 min challenge reading; 1 had a 1 + reaction, and 1 had a 2+ reaction. No other reactions were observed. The product was essentially nonirritating. A prophetic patch test (method of Swartz and Peck) and a repeated-insult patch test (method of Shelanski and Shelanski) was performed using a lipstick with 10-1 1 % Sesame Oil at 100% concentration. Of 102 panelists participating in the prophetic patch test, none had reactions with either closed or open patches, with or without ultraviolet (UV) light. Of 50 panelists in the repeated-insult patch test, none had reactions with either closed or open patches, with or without UV light (CTFA, 1989).
A prophetic patch test (method of Swartz and Peck) and a repeated insult patch test (method of Shelanski and Shelanski) was performed using a cuticle stick with 10-1 1 % Sesame Oil at 100% concentration. Of 104 panelists in the prophetic patch test, none had reactions with either closed or open patches, with or without UV light. Of 52 panelists in the repeated insult patch test, none had reactions with either closed or open patches, with or without UV light (CTFA, 1989).
Provocative
Ointments were prepared by mixing polymerized Sesame Oil and unpolymerized
Sesame Oil with petrolatum in a ratio of 1 :9 (w/w) (D’Souza and Ramaiah, 1980).
COSMETIC INGREDIENT REVIEW
Thirty-five volunteers with dermatitic lesions, consisting of thick and hyperpigmented skin and severe itching, participated in the study. Ten volunteers applied the unpoly merized Sesame Oil ointment twice daily for 2 years and 25 volunteers applied the polymerized Sesame Oil ointment twice daily for 2 years. All of the volunteers treated with the unpolymerized Sesame Oil ointment and two of the volunteers treated with the polymerized Sesame Oil ointment had no subjective or objective improvement. The itching decreased within 3 days in 23 of the volunteers treated with polymerized Sesame Oil ointment, and the itching completely stopped within 1 week and the skin was normal in appearance within 30 days in 19 of these volunteers. Of these 19 volunteers, 15 had no relapses within 2 years, and 4 had occasional relapses. Another 4 of the 23 volunteers had relief from itching when the polymerized Sesame Oil ointment was applied, but the appearance of the skin did not change even after 60 days of treatment.
No adverse reactions were reported. Crude Sesame Oil was extracted with methanol to prepare samples of sesarnin and
sesamolin (Neering et al., 1975). The sesamin and sesamolin samples were about 5-1 0% contaminated with each other. Thirteen patients with contact allergy to Sesame Oil were patch tested with 5% sesamol in methyl-ethyl-ketone ethanol or petrolatum, 5% methanol-extracted sesamin in methyl-ethyl-ketone or petrolatum, and 5% methanol-extracted sesamolin in methyl-ethyl-ketone or petrolatum. After 48 h, 8 patients had positive reactions to sesamol, 12 to sesamin, and 12 to sesamolin. Eight patients were patch tested with sesamol on thin-layer sheets wetted with methylethyl-ketone, and 12 patients were patch tested with sesamin and sesamolin on thin-layer sheets wetted with methyl-ethyl-ketone. After 48 h, two patients had positive reactions to sesamol, seven to sesamin, and four to sesamolin.
A woman with lipstickdermatitis was patch tested with Sesame Oil, and at 48 h had a positive reaction to the Sesame Oil. The woman was also patch tested with sesamin and sesamolin extracted from Sesame Oil and with sesamol. At 48 h, she had positive reactions to 0.1, 0.5, 1 .O, and 5.0% sesamin in petrolatum, and to 0.5, 1 .O, and 5.0% sesamolin in petrolatum. She had negative reactions to 0.1 % sesamolin in petrolatum,
and to 0.1, 0.5, 1 .O, and 5.0% sesarnol in petrolatum.
SUMMARY
Sesame Oil is primarily composed of oleic, linoleic, palmitic, and stearic acids. Three trace constituents-sesamol, sesamin, and sesamolin-are found only in Sesame Oil.
Sesame Oil is used in a variety of cosmetics that come into contact with skin, eyes, hair, nails, or mucous membranes. It is a GRAS direct food additive, and is used in pharmaceuticals. Sesame Oil and sesamol are used as synergists for pyrethrum insecticides.
In one experiment, injected radioactive Sesame Oil was detected in the heart, lungs, liver, and iliac lymph node. In other experiments in which Sesame Oil was injected subcutaneously, large amounts of the oil remained at the injection site throughout the experiment.
Sesamol weakly inhibited the hydroxylation of dimethylaminopyrine and hexobarbital. Sesamol and sesamin have a moderate effect on the prolongation of hexobarbital narcosis and zoxazolamine paralysis in mice.
The oral LD, of a lipstick containing 10-1 1 % Sesame Oil was greater than 5 g/kg.
SAFETY ASSESSMENT OF SESAME OIL 275
The dermal LD, was greater than 2 g/kg. The acute intravenous LD, of Sesame Oil for rabbits was 0.74 ml/kg.
The greatest primary irritation indices of Sesame Oil in 5 studies was 0.83 (maximum possible, 8.0).
Sesame Oil was not an ocular irritant.
Although not teratogenic, Sesame Oil increased resorptions and deciduomas in Extracts of sesame seeds were not mutagenic upon metabolic activation.
Numerous repeated-insult patch tests on normal volunteers reported a low irritancy potential for Sesame Oil. Patients with dermatitis treated with Sesame Oil for 2 years reported no adverse reactions. Of patients with contact allergy to Sesame Oil who were patch tested, the majority had positive reactions to sesamol, sesamin, and sesamolin. A woman with lipstickdermatitis had a positive reaction to the Sesame Oil.
DISCUSSION
In reviewing the information on Sesame Oil, the CIR Expert Panel noted the possibility of reactions to the trace constituents sesamol, sesamin, and sesamolin. One study linked high dosages of subcutaneously injected Sesame Oil to mammary adenocarcinomas in mice, but the results of this experiment were not duplicated by the investigator in a similar study.
CONCLUSION
On the basis of the information presented in this report, the CIR Expert Panel concludes that Sesame Oil is safe as a cosmetic ingredient in the present practices of use.
ACKNOWLEDGMENT Lynn Willis, former Scientific Analyst and Writer, prepared this report.
REFERENCES
ANTON, A.H., SERRANO, A,, BEYER, R.D., and LAVAPPA, K.S. (1974). Tetrahydro-cannabinol, sesame oil, and biogenic
BEAUDOIN, A.R. (1981). The failure of glutamic acid to protect the rat embryo against the action of trypan blue. Teratology
BLIZARD, D.A. (1978). The effect of neonatal oil administration on brain weight of inbred and heterogeneous mice. Dev.
BLUMENTHAL, H.T., and ROGERS, J.B. (1962). Studies of guinea pig tumors. II. The induction of malignant tumors in guinea pigs
BROWN, J.L. (1 983). Incomplete labeling of pharmaceuticals: A list of “inactive” ingredients. N. Engl. 1. Med. 309(7):439-41.
BUDOWSKI, P., and MARKLEY, K.S. (1951). The chemical and physiological properties of sesame oil. Chem. Rev. 48:125-51.
BULAY, O.M. (1 970). The study ofdevelopment of lung and skin tumors in mice exposed In utero to polycyclic hydrocarbons. Acta
amines. Life Sci. 14(9):1741-6.
23(1):95-9.
Psychobiol. 11(5):487-93.
by methyl-cholanthrene. Cancer Res. 221 155-62.
Med. Turc. 7(1):3-38.
276 COSMETIC INGREDIENT REVIEW
BULAY, O.M., and WATTENBERC, L.W. (1971). Carcinogenic effects of poly-cyclic hydrocarbon carcinogen administration to
CHECCHI, A.A. (1980-1983). FDA OTC Drug Ingredient Index and Manual.
CODE OF FEDERAL REGULATIONS (CFR). (1984). Title 21. Food and Drugs. Washington, D.C.: U.S. Government Printing
COSMETIC, TOILETRY AND FRAGRANCE ASSOCIATION. (CTFA). (1 972a). Primary skin irritation; test no. S3/233. CTFA code
CTFA. (1972b). Eye irritation; test no. E14/1552. CTFAcode no. 3-27-2, November 14.’
CTFA. (1972~). Clinical evaluation report; profile no. 40065. CTFA code no. 3-27-3, November 22.’
CTFA. (1 975a). Primary skin irritation; test no. 58/35, CTFA code no. 3-27-1, August 4.’
CTFA. (1975b). Primary skin irritation; test no. 58/45, CTFA code no. 3-27-1, August 25.’
CTFA. (1 975c). Primary skin irritation; test no. 58/70, CTFA code no. 3-27-1, November 5.’
CTFA. (1 975d). Eye irritation; test no. E26/47. CTFA code no. 3-27-2, November 7.’
CTFA. (1 976). Allergic contact sensitization test; tesl no. 010-76, profile no. 9467. CTFA code no. 3-27-6, November 1 .’
CTFA. (1 978). Primary skin irritation; test no. 911 80. CTFA code no. 3-27-1, February 27.’
CTFA. (1 989). Primary skin irritation, repeated insult patch tests, phototoxicity, LD, dermal toxicity, ocular irritation, March 23.’
CTFA. (1 992). UV absorption spectra, January 7.’
DAHL, A., and BREZINSKI, D. (1 985). Inhibition of rabbit nasal and hepatic cytochrome P-450-dependent hexamethylphosphoraD’SOUZA, C.J.M., and RAMAIAH, T.R. (1980). Effect of topical application of sesame oil polymer on skin. Indian 1. Med. Res.
DRILL, V.A., and LAZAR, P. (eds.) (1 977). The chamber-scarification test for assessing irritancy of topically applied substances. In:
ESTRIN, N.F., CROSLEY, P.A., and HAYNES, C.R. ieds.1 (1982a). CTFA Cosmetic Ingredient Dictionary, 3rd ed. Washington,
ESTRIN, N.F., HAYNES, C.R., and WHELAN, 1.M. (eds.) (1982b). CTFA Compendium of Cosmetic Ingredient Composition:
FENNELL, T., and BRIDGES, 1. (1979). Structure-activity relationship for ’safrole-type’ cytochrome P-450 induction. Biochem.
FOOD AND DRUG ADMINISTRATION (FDA) (1 987). Cosmetic Product Formulation Data. Washington, D.C.
FDA (1 991). Cosmetic Product Formulation Data. Washington, D.C.
FUIII, K., IAFFE, H., BISHOP, Y., ARNOLD, E., MACKINTOSH, D., and EPSTEIN, S. (1970). Structure-activity relations for
methylenedioxyphenyl and related compounds on hepatic microsomal enzyme function, as measured by prolongation of
hexobarbital narcosis and zoxazolamine paralysis in mice. Toxicol. Appl. Pharmacol. 16482-94.
CENEROSO, W.M., CAIN, K.T., HOSKINS, I.A., WASHINGTON, W.I., and RUTLEDCE, I.C. (1 984). Pseudo dominant-lethal
response in female mice treated with plant oils. Mutat. Res. 129:235-41.
COSSELIN, R.E., HODCE, H.C., SMITH, R.P., and CLEASON, M.N. (1 976). Clinical Toxicology of Commercial Products: Acute
Poisoning, 4th ed. Baltimore: Williams and Wilkins.
HAYAKAWA, R., MATSUNACA, K., SUZUKI, M., HOSOKAWA, K., ARIMA, Y., SHIN, C.S., and YOSHIDA, M. (1987). Is
sesamol present in sesame oil! Contact Derm. 17:133-35.
HILL TOP RESEARCH (1976a). Submission of unpublished data by CTFA. Repeated insult patch test of ten test materials; test no.
76-331 -72, lune 30.’
HILL TOP RESEARCH (1976b). Submission of unpublished data by CTFA. Repeated insult patch test; test no. 75-867-72. January
26.
HILL TOP RESEARCH (1977). Submission of unpublished data by CTFA. Repeated insult patch test of ten test materials; test no.
76-1039-72, February 22.’
HIRAYAMA, T., YAMADA, N., NOHARA, M., and FUKUI, S. (1 984). The existence of the 1,2-dicarbonyl compounds glyoxal,
methyl glyoxal and diacetyl in autoxidized edible oils. I. Sci. Food Agric. 35(12):1357-62.
HIROSE, M., MASUDA, A., IMAIDA, K., KACAWA, M., TSUDA, H., and ITO, N. (1 987). Induction offorestomach lesions in rats
by oral administrations of naturally occurring antioxidants for 4 weeks. Jpn. 1. Cancer Res. 78(4):317-21.
HIROSE, M., FUKUSHIMA, S., SHIRAI, T., HASECAWA, R., KATO, T., TANAKA, H., ASAKAWA, E., and ITO, N. (1990).
Stomach carcinogenicity of caffeic acid, sesamol, and catechol in rats and mice. Ipn. I. Cancer Res. 81(3):207-12.
HUCCINS, C., and GRAND, L.C. (1963). Sarcoma induced remotely in rats fed 3-methylcholanthrene. Cancer Res.
23(3):477-80.
mice during pregnancy on the progeny. 1. Natl. Cancer Inst. 46(2):397-402.
Office.
no. 3-27-1, April 16.’
mide (HMPA) N-demethylase by methylenedioxyphenyl compounds. Biochem. Pharmacol. 34(5):631-6.
72:120-7.
Cutaneous Toxicity. New York: Academic Press, pp. 127-54.
D.C.: The Cosmetic, Toiletry and Fragrance Association.
Specifications/Spectra. Washington, D.C.: Cosmetic, Toiletry and Fragrance Association.
SOC. Trans. 7(5):1104-6.
’Available for review: Director, Cosmetic Ingredient Review, 1101 17th Street, N.W., Suite 310, Washington, DC 20036.
SAFETY ASSESSMENT OF SESAME OIL 277
JAFFE, H., FUIII, K., SENCUPTA, M., CUERIN, H., and EPSTEIN, S. (1968). In VIVO inhibition of mouse liver microsomal
JONES, L.A , and BERN, H.A. (1 979). Cervicovaginal and mammarygland abnormalities in BALB/CCRCL mice treated neonatally
KURECHI, T., KIKUCAWA, K., and KATO, T. (1 979). C-nitrosation of sesamol and its effects on N-nitrosamine formation in vitro.
KURECHI, T., KIKUCAWA, K., and NISHIZAWA, A. (1 9801. Transformation of hemoglobin A Into methernoglobin by sesamol.
NEERINC, H., VITANYI, B.E., MALTEN, K.E., van KETEL, W.C., and van DIIK, E. (19751. Allergens in sesame oil contact
NIKITAKIS, J. (1988). The CTFA Cosmetic Ingredient Handbook. Washington, D.C.: CTFA, p. 370.
ROCKWELL, P., and RAW, I. (1 979). Mutagenic screening of various herbs, spices and food additives. Nutr. Cancer 1(4):10-5.
ROSSOFF, I.S. (1974). Handbook of Veterinary Drugs. A Compendium for Research and Clinical Use. New York: Springer
SENCUPTA, P.M., and ROY, B.R. (1983). Aflatoxin content in edible oils and fats. I. Inst. Chem. (India) 55(3):101-4.
SHEPPARD, A.I., IVERSON, I.L., and WEIHRAUCH, J.L. (1978). Composition ofselected dietary fats, oils, margarines, and butter.
SVENDSEN, O., and AAES-JORCENSEN, T. (1979). Studies on the fate of vegetable oil after intramuscular injection into
SWERN, D. (ed.) (1979). Bailey’s Industrial Oil and Fat Products, Vol. 1, 4th ed. New York: John Wiley and Sons.
SZEPSENWOL, I., FLETCHER, I., MURISON, C.L., and TORO-COYCO, E. (1979a). Long term effects of delta-9-tetrahydrocanSZEPSENWOL, I., FLETCHER, I., and TORO-COYCO, E. (1979b). Tumors in mice receiving subcutaneous injections of delta-9-
SZEPSENWOL, I., FLETCHER, I., and TORO-COYCO, E. (1979~). Mammary cancer in mice receiving weekly subcutaneous
SZEPSENWOL, I., FLETCHER, I., and CASALES, E.A. (1982). Adrenal cortical tumorigenesis in delta-tetrahydrocannabinol (THC)
WINDHOLZ, M. (ed.) (1983). The Merck Index, 10th ed. Rahway, Newlersey: Merck and Co.